Extraction and Socket Grafting: Part 3 — Socket Grafting Protocol
In Part 1 (Chairside® magazine; Vol. 14, Issue 1) of this series, atraumatic extraction and maintenance of hard and soft tissues were discussed. Part 2 (Chairside; Vol. 14, Issue 2) examined extraction site healing, as well as indications and contraindications for socket grafting. In this third and final part of the series, a step-by-step socket grafting protocol will be explained, including various techniques that are specific to the anatomy of the particular extraction socket.
Numerous socket-grafting techniques have been discussed in the literature; however, most clinicians today still utilize a single technique for socket grafting, without regard for the number of walls of bone remaining after tooth extraction. To achieve a consistent and successful outcome, clinicians must be able to recognize the various types of extraction sockets and understand the need to implement a specific treatment protocol for each. They must also utilize the ideal graft material and barrier membrane for each socket type. The following step-by-step protocol will discuss the surgical techniques for the most common types of extraction sockets, including five-walled, four-walled and three-walled sites, and procedures to maximize the success of the bone regeneration process.
DETERMINING THE TYPE OF MATERIAL TO USE
1. Bone Graft Material
In the literature, there is no consensus with respect to a generalized and ideal type of bone grafting material and barrier membrane. Most studies completed on types of bone graft materials include either allograft or xenograft bone substitutes. Allografts are classified as either demineralized (DFDBA) or mineralized (FDBA) freeze-dried bone allograft. Because of the remaining calcium and phosphate salts, FDBA tends to resorb much slower, and therefore maintains space much better than DFDBA. On the other hand, demineralized freeze-dried bone has osteoinductive qualities, but resorbs much faster, with inconsistent results when used as the sole bone grafting material.1 Piattelli et al. compared DFDBA and FDBA and reported FDBA to be a better scaffolding material with greater osteoconductive qualities.2

Xenografts have been found to produce varying results in extraction sites. Most studies show inconsistent resorption, in some cases taking years to turn over.3,4 Therefore, for extraction sites, a mineralized allograft tends to be more consistent and ideal. Based on this fact, the author has recommended that most sockets be bone-grafted with mineralized freeze-dried allograft, such as Newport Biologics™ Mineralized Cortico/Cancellous Allograft Blend (Glidewell Direct; Irvine, Calif.) or a 70%/30% combination of mineralized and demineralized bone. Table 1 describes the amount of material typically required for various extraction sites.
2. Barrier Membrane
Because soft-tissue cells migrate at a faster rate than bone-forming cells, a biocompatible barrier membrane is recommended in conjunction with the socket graft procedure to prevent soft-tissue invasion. Many studies have concluded that the use of a barrier membrane in socket grafts reduces the amount of crestal and horizontal ridge resorption5 In general, the larger the socket or the more missing walls, the longer-acting the membrane should be for that site.
There are two types of membranes available for socket grafting: resorbable (e.g., collagen) and non-resorbable (e.g., PTFE). If all walls of the extraction socket are intact (five-walled socket), but are insufficient in thickness (less than 1.5 mm), then a faster-resorbing collagen (e.g., Newport Biologics Resorbable Collagen Plug) is used over the socket. If the buccal plate is missing (four-walled socket) or a larger defect is present, a longer-acting collagen (e.g., Newport Biologics Resorbable Collagen Membrane 4-6) or a PTFE membrane (e.g., CytoSurg™ Non-Resorbable PTFE Membrane [Salvin Dental Specialties; Charlotte, N.C.]) is used. The collagen membrane is advantageous as it is resorbable and does not require removal. The PTFE membrane can be exposed to the oral cavity, and its use decreases the risk of bacterial contamination while preventing the ingrowth of soft tissue. However, PTFE membranes require a surgical procedure for removal. Various bone substitutes and membrane types and indications were discussed in the Chairside magazine articles “Bone Substitutes in Oral Implantology” (Vol. 12, Issue 3) and “The Use of Barrier Membranes in Implant Dentistry” (Vol. 13, Issue 1).
If soft-tissue augmentation is required because of insufficient attached tissue, the use of an acellular dermal matrix, such as OrACELL® Decellularized Dermis (LifeNet Health; Virginia Beach, Va.), may be used. These collagen materials are advantageous as they enhance the soft tissue, may be left exposed to the oral cavity, and act as an excellent membrane to prevent soft-tissue invasion.
SOCKET GRAFT PROTOCOL
The proper grafting protocol can be applied from determining the number of socket walls remaining after extraction.
1. Five-Walled Extraction Socket (Less Than 1.5 mm Buccal Wall Thickness)
When a five-walled socket is present with a buccal plate of less than 1.5 mm, it is ideal to complete a socket graft procedure. Because of the compromised thickness of the buccal wall, the added bone graft material will allow for space maintenance and a more predictable regenerative process.
Five-Walled Socket Bone Grafting Technique (Figs. 1a–1d, 2a–2d)
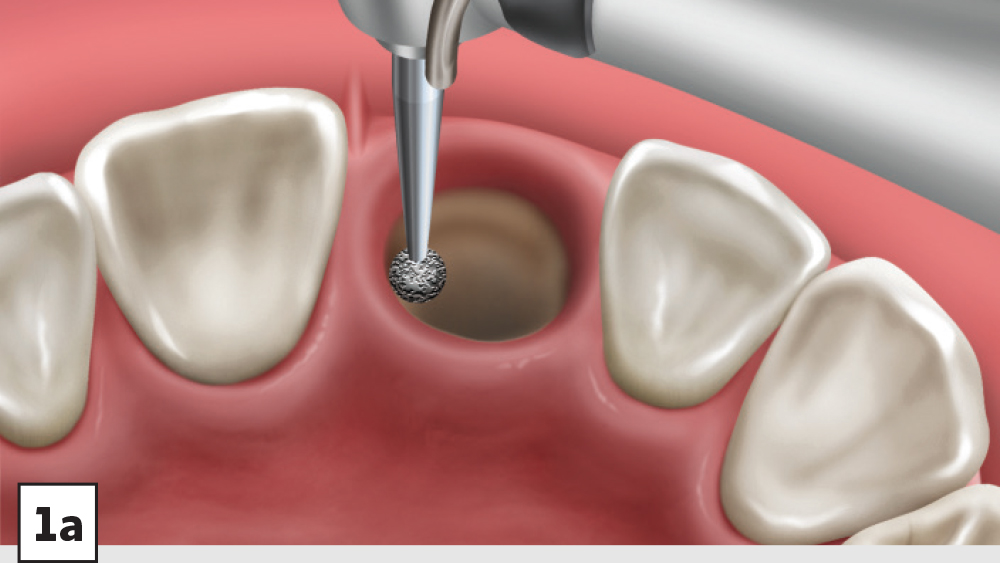
Figures 1a–1d: Five-walled socket: The bone grafting protocol involves the removal of all soft-tissue remnants and decortication (1a), bone graft placed into the socket at the level of the bony crest (1b), one-half collagen plug placed over the bone graft (1c), and a crisscross suture (1d).
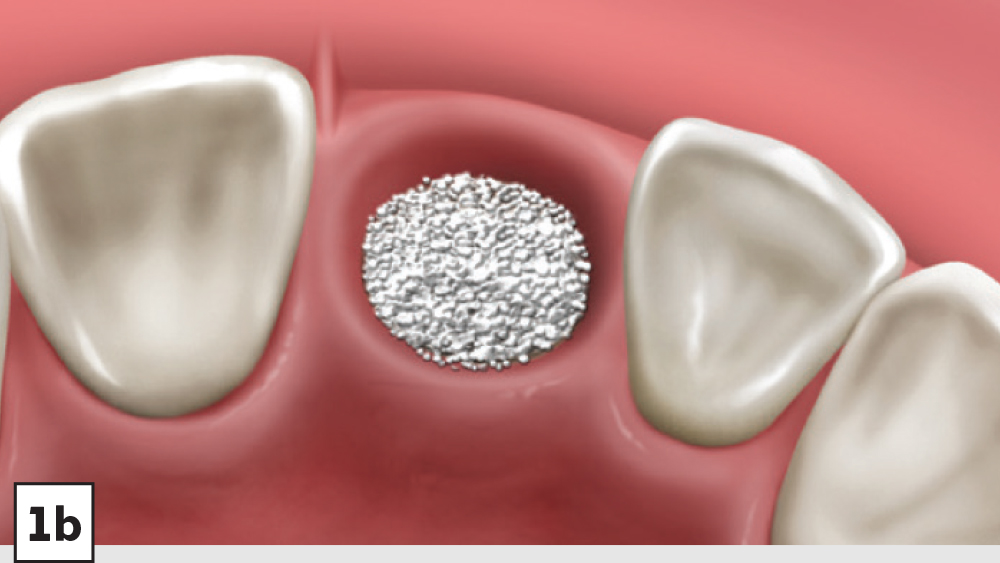
Figures 1a–1d: Five-walled socket: The bone grafting protocol involves the removal of all soft-tissue remnants and decortication (1a), bone graft placed into the socket at the level of the bony crest (1b), one-half collagen plug placed over the bone graft (1c), and a crisscross suture (1d).
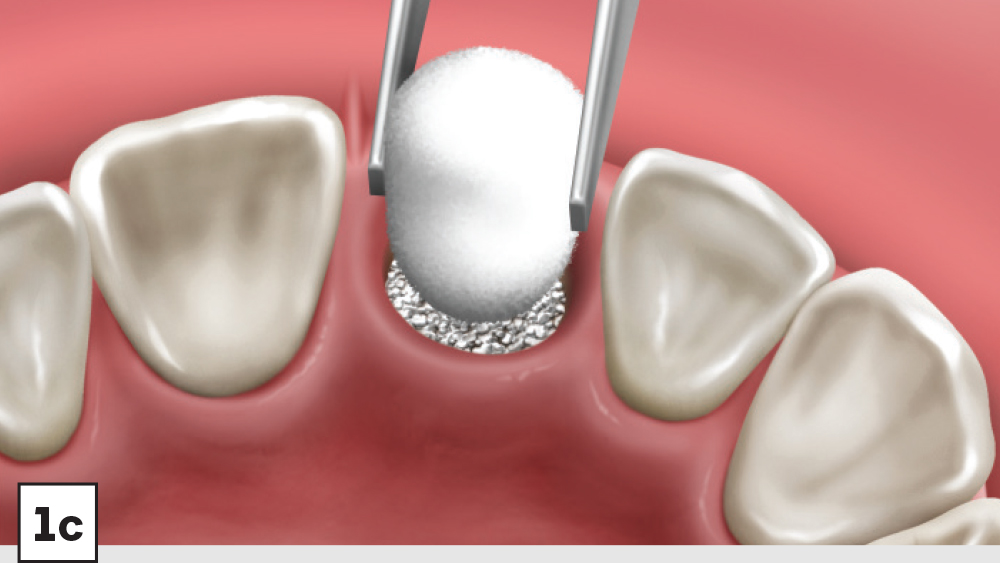
Figures 1a–1d: Five-walled socket: The bone grafting protocol involves the removal of all soft-tissue remnants and decortication (1a), bone graft placed into the socket at the level of the bony crest (1b), one-half collagen plug placed over the bone graft (1c), and a crisscross suture (1d).
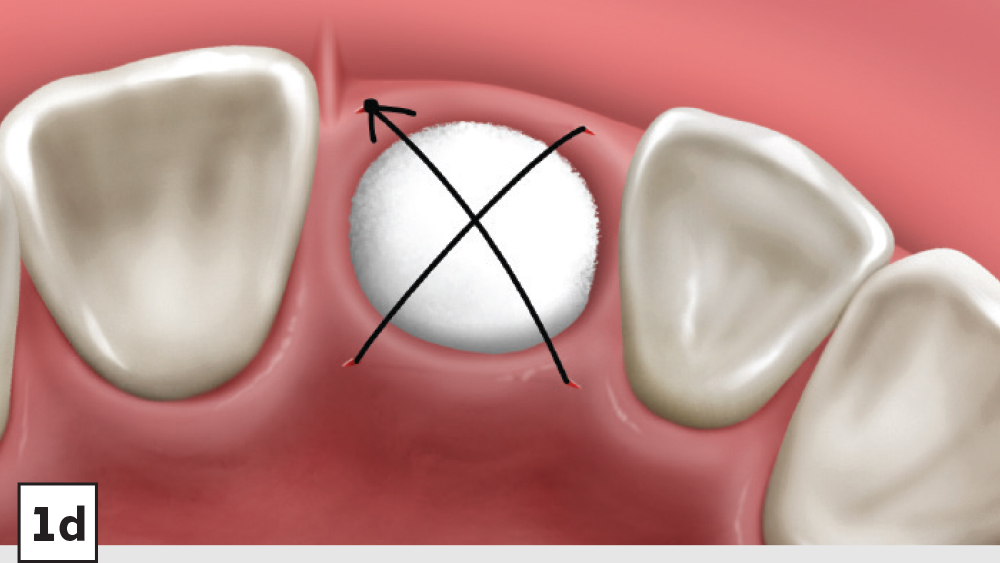
Figures 1a–1d: Five-walled socket: The bone grafting protocol involves the removal of all soft-tissue remnants and decortication (1a), bone graft placed into the socket at the level of the bony crest (1b), one-half collagen plug placed over the bone graft (1c), and a crisscross suture (1d).
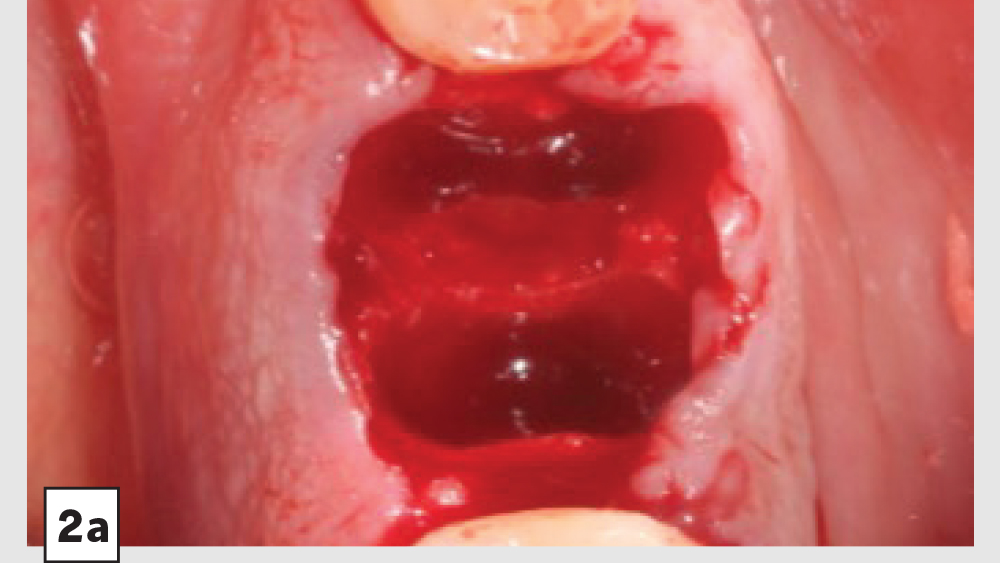
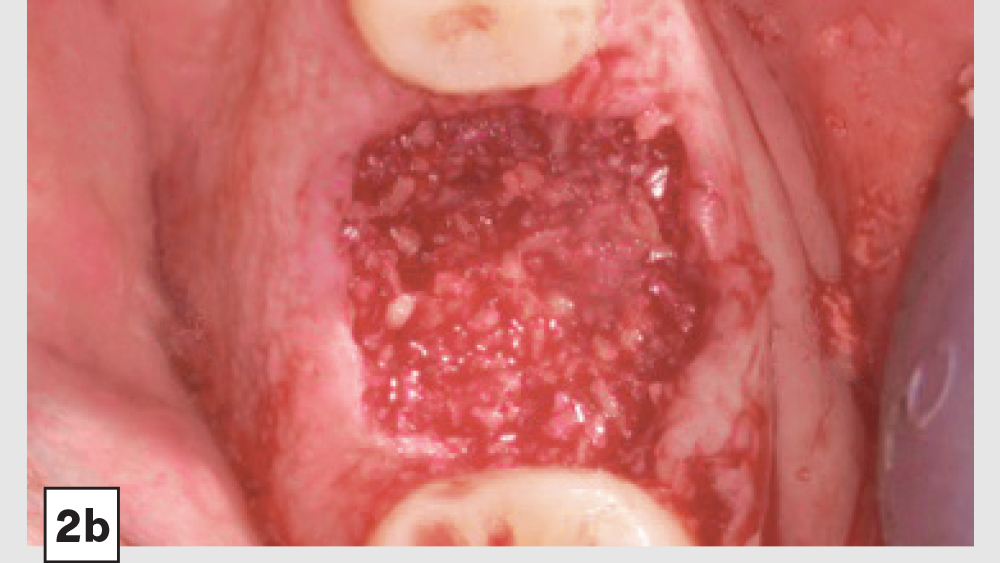
Figures 2a–2d: Five-walled socket: Extraction of mandibular first molar (2a), placement of Newport Biologics Cortico/Cancellous Allograft Blend (2b), Newport Biologics Resorbable Collagen Plug (2c), collagen plug cut in half and placed over graft (2d).
Figures 2a–2d: Five-walled socket: Extraction of mandibular first molar (2a), placement of Newport Biologics Cortico/Cancellous Allograft Blend (2b), Newport Biologics Resorbable Collagen Plug (2c), collagen plug cut in half and placed over graft (2d).

Figures 2a–2d: Five-walled socket: Extraction of mandibular first molar (2a), placement of Newport Biologics Cortico/Cancellous Allograft Blend (2b), Newport Biologics Resorbable Collagen Plug (2c), collagen plug cut in half and placed over graft (2d).
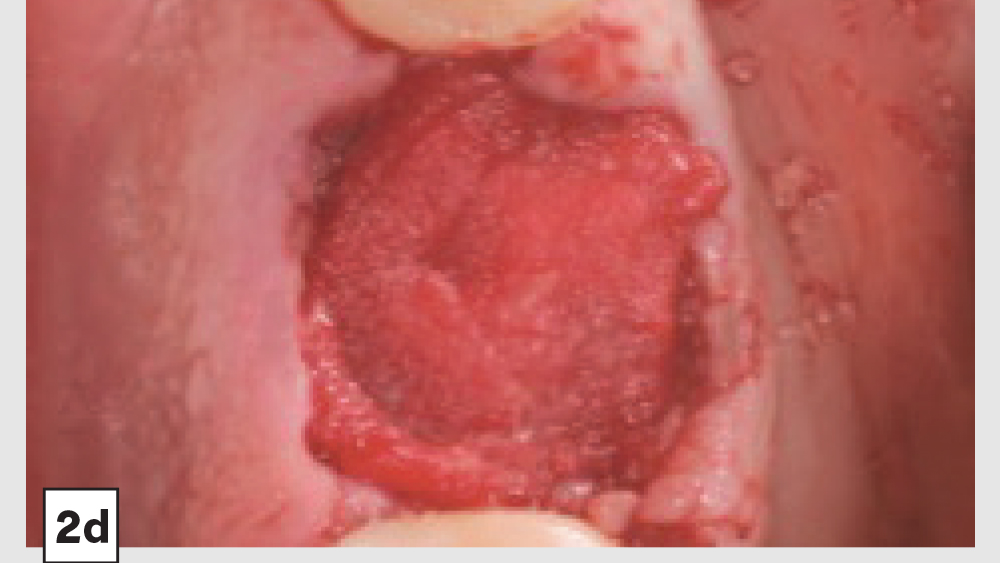
Figures 2a–2d: Five-walled socket: Extraction of mandibular first molar (2a), placement of Newport Biologics Cortico/Cancellous Allograft Blend (2b), Newport Biologics Resorbable Collagen Plug (2c), collagen plug cut in half and placed over graft (2d).
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- Reflection: After extraction, a mucoperiosteal flap should not be reflected in most circumstances. However, a small subperiosteal pouch may be made around the margins of the extraction socket to allow for ease of membrane placement and suturing.
- Preparation: The extraction socket should be free of soft-tissue remnants and bleeding should be present. If bleeding is not present, the remaining walls should be decorticated. Care should be exercised around adjacent teeth or vital structures. Bleeding from decortication will facilitate early vascularization and initiate the healing process.
- Graft Material: The graft material (e.g., Newport Biologics Mineralized Cortico/Cancellous Allograft Blend) should be hydrated with sterile saline (0.9% sodium chloride) or platelet-rich fibrin (PRF) and then gently condensed into the socket. Small increments of material should be added into the socket, and a bone-packing instrument, such as the Bone Carrier and Spoon included with the 12-piece Newport Biologics Implant and Bone Grafting Instrument Kit (Glidewell Direct), should be utilized to condense the material to avoid air spaces (Fig. 3). Usually, when “push-back” of the material is present, the socket is packed sufficiently. Care should be exercised to avoid packing the graft material too densely, as this may interfere with angiogenesis and delay the healing process.

Figure 3: The Newport Biologics Implant and Bone Grafting Instrument Kit includes the most commonly used instruments to streamline and complete bone grafting procedures.
- Membrane: A Newport Biologics Resorbable Collagen Plug should be cut in half and hydrated with 0.9% sodium chloride. The plug is compressed and placed over the socket, extending the collagen underneath the reflected subperiosteal pouch to increase retention.
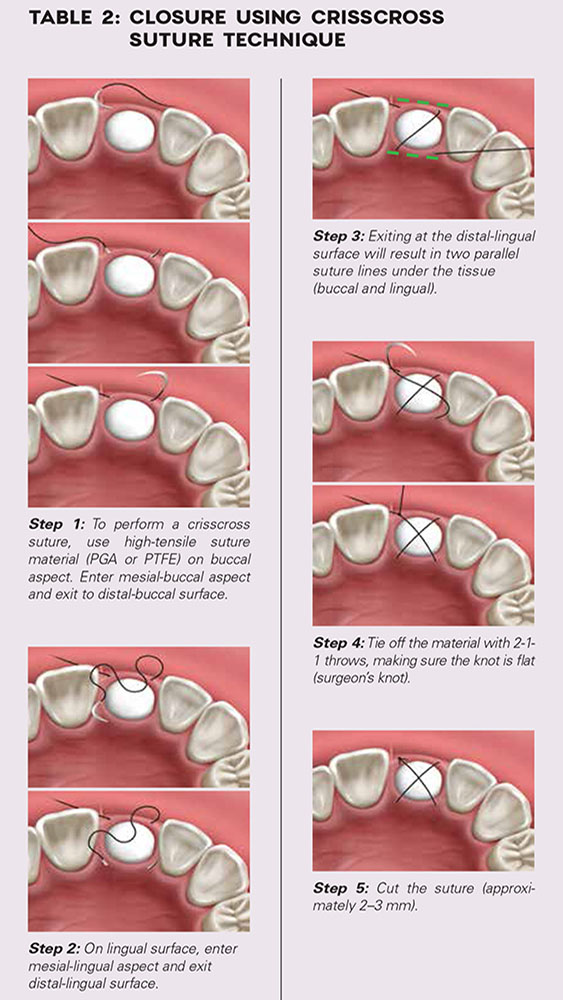
- Closure: Closure should be completed with a high-tensile suture material, such as polyglycolic acid (PGA) or PTFE, with a crisscross suture technique (Table 2). Care should be exercised to avoid suturing through the membrane. Gut sutures (plain, chromic) should be avoided, as they have a compromised tensile strength and will increase the probability of bone graft and membrane loss.
2. Four-Walled Extraction Socket
Most often in a four-walled socket, the buccal (labial) wall is lost. When the buccal plate is missing and not treated properly, the absence of the wall prevents space maintenance, reduces host bone vascularization, allows for the migration of bone graft material, and increases the possibility of soft-tissue invasion. Therefore, a different surgical protocol is recommended that includes the use of a longer-acting membrane over the missing buccal plate, which will maintain the space and prevent loss of bone material.
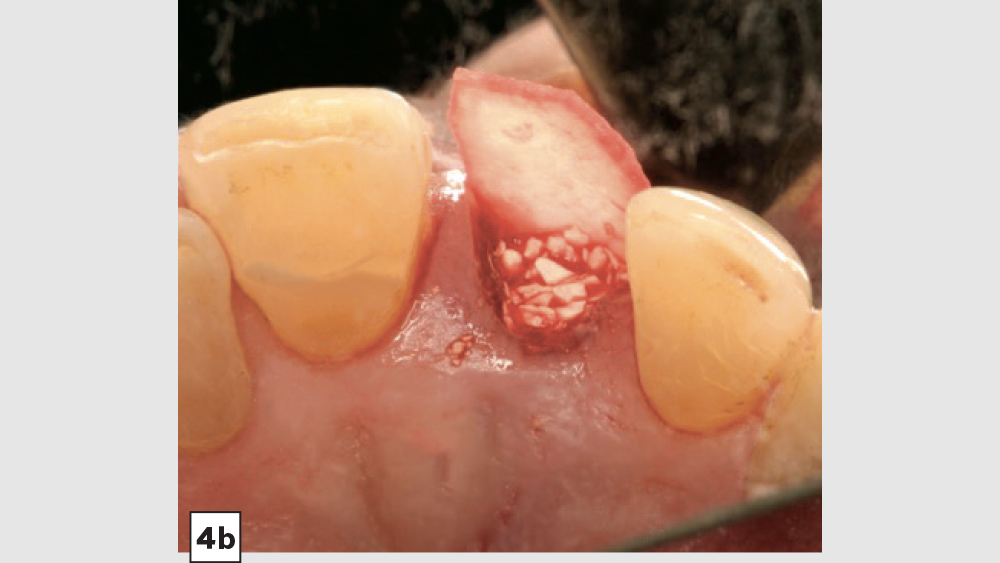
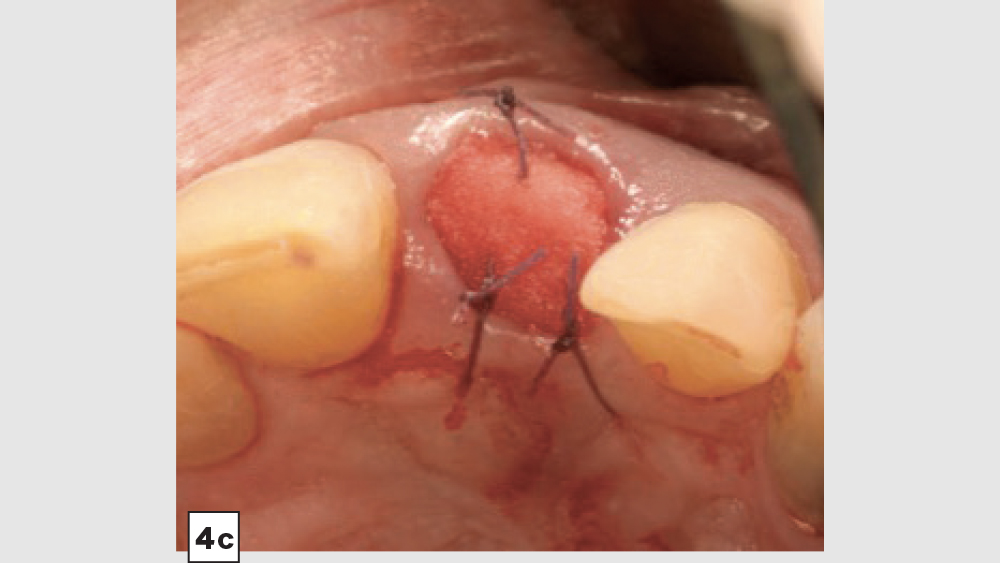
Four-Walled Socket Bone Grafting Technique (Figs. 4a–4d, 5a–5c)
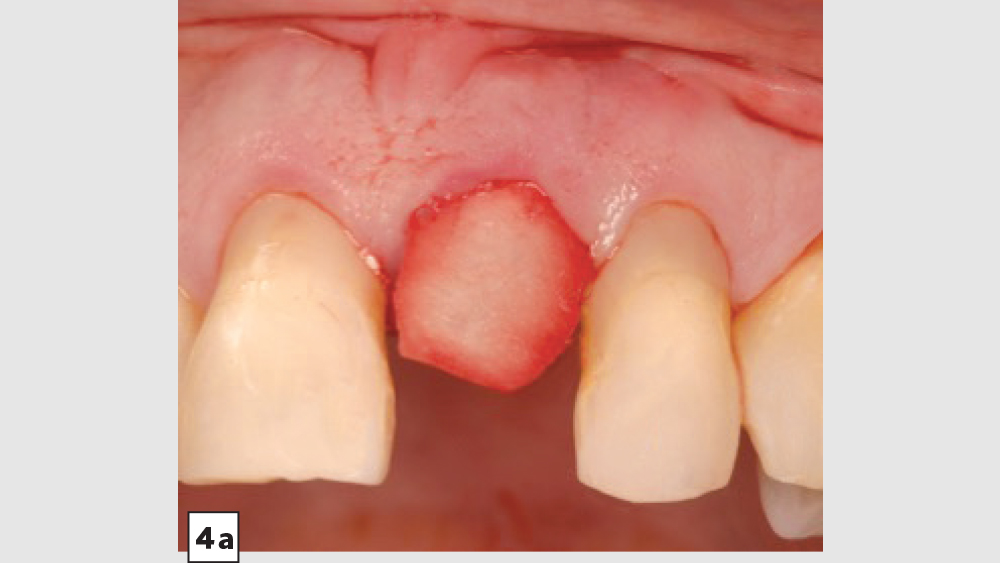
Figures 4a–4d: Four-walled socket: Newport Biologics Resorbable Collagen Membrane 4-6 placed over buccal plate (4a), socket grafting with Mineralized Cortico/Cancellous Allograft Blend (4b), and closure (4c). Shown is the site at four weeks of healing (4d).
Figures 4a–4d: Four-walled socket: Newport Biologics Resorbable Collagen Membrane 4-6 placed over buccal plate (4a), socket grafting with Mineralized Cortico/Cancellous Allograft Blend (4b), and closure (4c). Shown is the site at four weeks of healing (4d).
Figures 4a–4d: Four-walled socket: Newport Biologics Resorbable Collagen Membrane 4-6 placed over buccal plate (4a), socket grafting with Mineralized Cortico/Cancellous Allograft Blend (4b), and closure (4c). Shown is the site at four weeks of healing (4d).
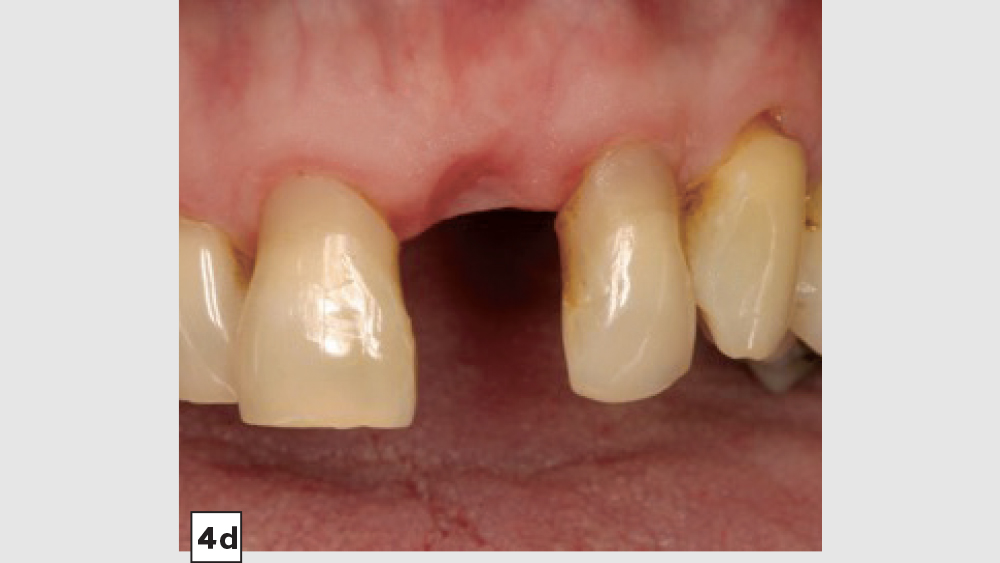
Figures 4a–4d: Four-walled socket: Newport Biologics Resorbable Collagen Membrane 4-6 placed over buccal plate (4a), socket grafting with Mineralized Cortico/Cancellous Allograft Blend (4b), and closure (4c). Shown is the site at four weeks of healing (4d).
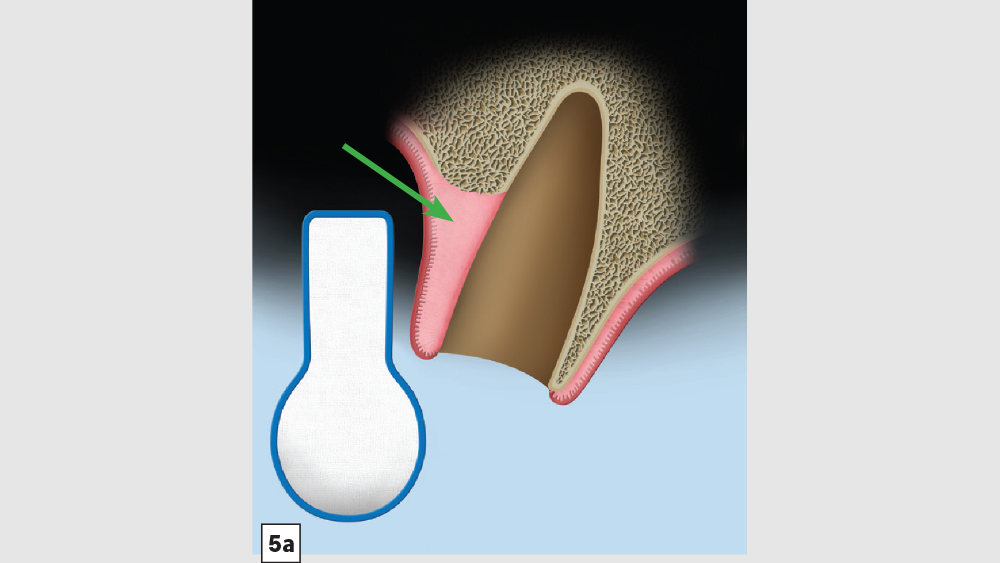
Figures 5a–5c: Membrane placement technique for four-walled socket (missing buccal plate): The protocol involves the membrane trimmed in a modified V-shape (5a). The membrane is placed to cover the missing buccal plate (5b) and then tucked under the lingual flap (5c).
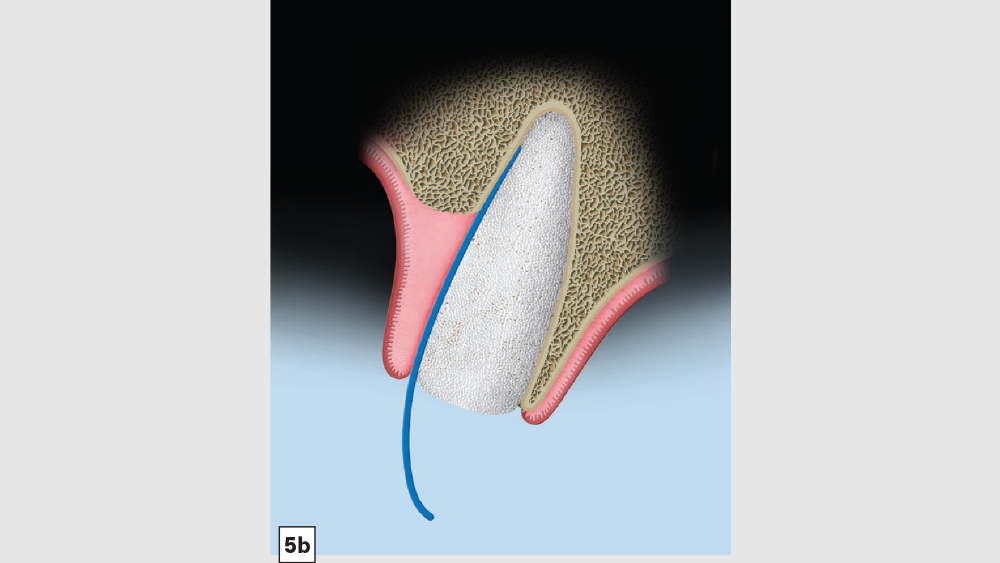
Figures 5a–5c: Membrane placement technique for four-walled socket (missing buccal plate): The protocol involves the membrane trimmed in a modified V-shape (5a). The membrane is placed to cover the missing buccal plate (5b) and then tucked under the lingual flap (5c).
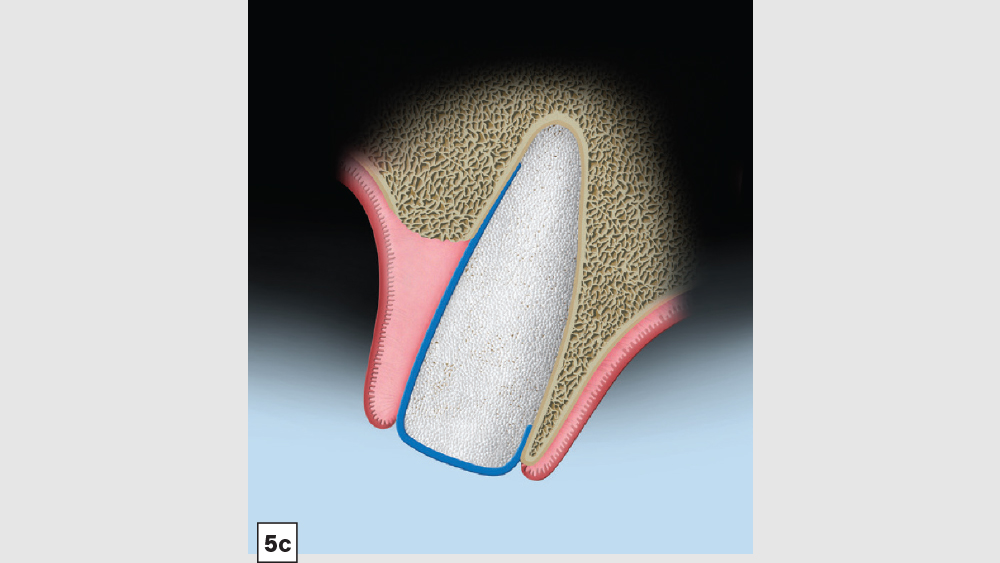
Figures 5a–5c: Membrane placement technique for four-walled socket (missing buccal plate): The protocol involves the membrane trimmed in a modified V-shape (5a). The membrane is placed to cover the missing buccal plate (5b) and then tucked under the lingual flap (5c).
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- Reflection: In most four-walled sockets, a partial mucoperiosteal flap should be reflected to allow for surgical access to the missing buccal plate. This will allow the clinician to place a membrane properly along the buccal plate.
- Preparation: Ensure there are no soft-tissue remnants within the socket and that bleeding is present. If bleeding is not present, the remaining walls should be decorticated. Care should be exercised around adjacent teeth or vital structures. Bleeding will facilitate early vascularization and initiate the healing process.
- Graft Material: The graft material (e.g., Newport Biologics Mineralized Cortico/Cancellous Allograft Blend) should be hydrated with sterile saline (0.9% sodium chloride) or platelet-rich fibrin and then gently condensed into the socket. Only small increments of material should be entered into the socket at a time, and a bone-packing instrument should be utilized to condense the bone graft material to avoid air spaces. Usually, when “push-back” of the material is present, the material is packed sufficiently. Care should be exercised to avoid packing the graft material too densely, as this may displace the membrane, interfere with angiogenesis and delay the healing process.
- Membrane: Because of the missing buccal plate, a longer-acting collagen cross-linked membrane (e.g., Newport Biologics Resorbable Collagen Membrane 3-4) or a PTFE (e.g., CytoSurg Non-Resorbable PTFE Membrane) should be used. The membrane should be trimmed in a modified V-shape (Fig. 5a).6 The narrower part of the membrane should encompass the entire missing buccal wall. Placement of the membrane should be within the confines of the socket, as extending the membrane over the external aspect of any remaining buccal wall will compromise the blood supply. The membrane should cover only the missing buccal wall; the other walls should not have membrane coverage, as this will decrease the healing of the socket. The goal of the membrane is to prevent the soft tissue from repopulating the defect. The wider part of the membrane should be trimmed so that it is slightly larger than the socket opening to allow for placement under the flap.
- Closure: Closure should be completed with a 3-0 or 4-0 high-tensile suture material (e.g., PGA or PTFE) with a crisscross suture technique. Care should be exercised to avoid suturing through the membrane. Gut sutures (plain, chromic) should be avoided, as they have a compromised tensile strength and incision line breakdown may occur.
3. Three-, Two- and One-Walled Extraction Sockets
When multiple socket walls of bone are missing, there is a greater need for the use of autogenous bone along with an allograft material. In implant dentistry today, these types of defects are commonly grafted solely with allograft material to simplify the surgical procedure. However, with these types of compromised extraction sockets, a particulate graft alone can be very unpredictable. For most three-walled defects, a donor site is most often used to obtain autogenous bone — typically the mandible (i.e., ramus, symphysis) or maxilla (i.e., tuberosity). In some cases, a membrane tent screw may be indicated to increase space maintenance and predictability.
Two- and one-walled sockets are very rare and usually will require autogenous block grafting. In addition, biologic growth factors (platelet-rich fibrin [PRF], platelet-rich plasma [PRP], and bone morphogenic protein [BMP]) are often used to increase the predictability and success of the bone graft.
Autogenous Grafting: Secondary Sites
a. Mandibular Ramus Donor Site — The “Scraping Technique”: One option to obtain autogenous bone is to expose the mandibular ramus and remove bone from the external oblique ridge with double-action rongeurs (Figs. 6a, 6b). The “scrapings” may be placed in a surgical bowl with sterile saline. Ramus block grafts may be harvested; however, the blocks need to be reduced into smaller pieces, which is rather time-consuming. Additionally, a ramus block graft (i.e., veneer graft of the lateral ramus) has a higher surgical morbidity and far greater post-op complications.
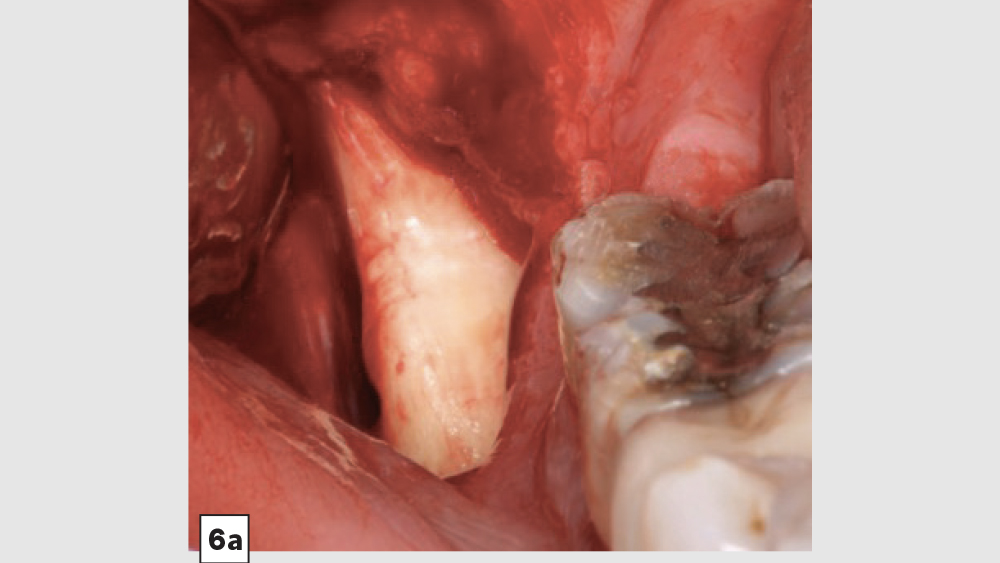
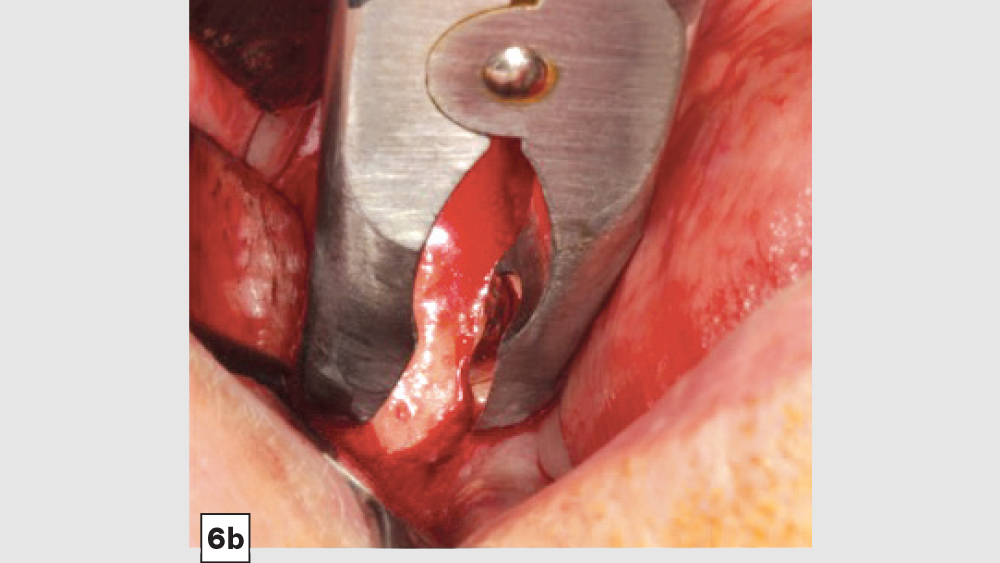
Figures 6a, 6b: Ramus autogenous graft: mandibular ramus exposed (6a), scrapings harvested with double-action rongeurs (6b).
b. Mandibular Ramus Donor Site — “Trephine Bur Bone Harvest”: A second method to harvest autogenous bone from the mandibular ramus is the use of trephine burs (Figs. 7a–7d). Trephine burs are end-cutting burs that are available in various diameters, with the 6–8 mm trephine being the most popular for harvesting bone from the ramus. One half of the trephine bur is placed over the external oblique bony ridge, while the other half is lateral to the bone and above the reflected masseter muscle, which is elevated off the anterior lateral aspect of the ramus. The trephine bur is used with an angled surgical drill at 2,000 rpm with copious saline irrigation, to a depth of approximately 5–8 mm, making sure the cuts are above and lateral to the position of the inferior alveolar nerve (IAN), artery and vein. The IAN position should be identified via a CBCT exam survey.

Figures 7a–7d: Trephine technique: trephine bur in latched handpiece (7a), multiple trephine osteotomies that overlap each other (7b), removal of autograft from ramus (7c), harvested autogenous bone chips to be used for socket grafting (7d).
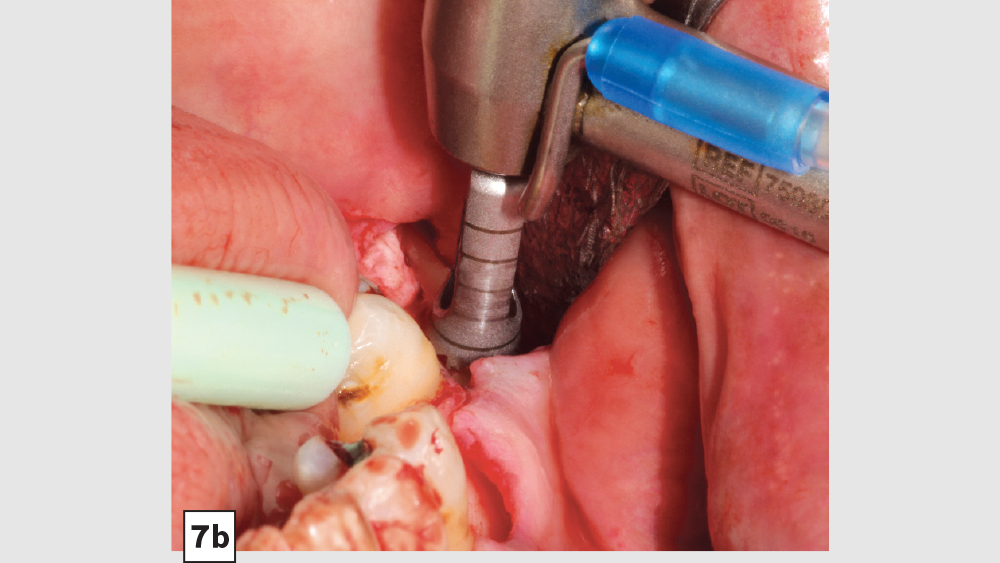
Figures 7a–7d: Trephine technique: trephine bur in latched handpiece (7a), multiple trephine osteotomies that overlap each other (7b), removal of autograft from ramus (7c), harvested autogenous bone chips to be used for socket grafting (7d).
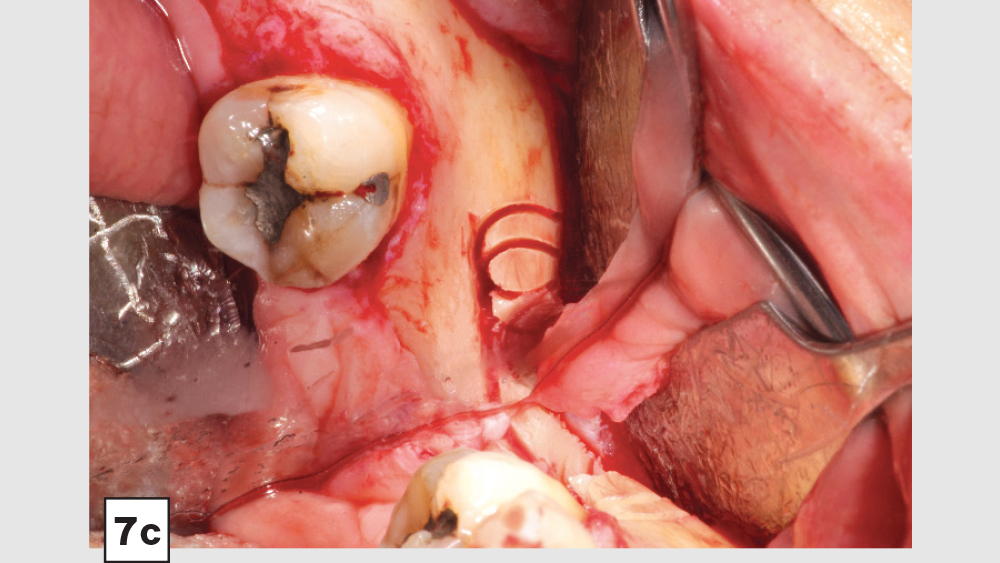
Figures 7a–7d: Trephine technique: trephine bur in latched handpiece (7a), multiple trephine osteotomies that overlap each other (7b), removal of autograft from ramus (7c), harvested autogenous bone chips to be used for socket grafting (7d).
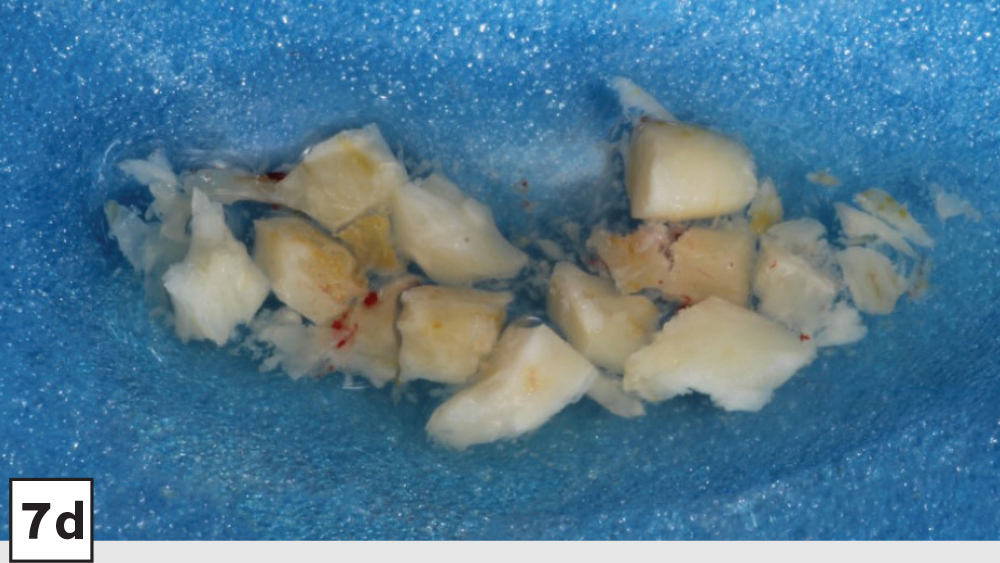
Figures 7a–7d: Trephine technique: trephine bur in latched handpiece (7a), multiple trephine osteotomies that overlap each other (7b), removal of autograft from ramus (7c), harvested autogenous bone chips to be used for socket grafting (7d).
c. Maxillary Tuberosity Donor Site: The maxillary tuberosity offers a variable amount of trabecular bone, which is dependent on the extent of maxillary bone atrophy and maxillary sinus pneumatization. The cancellous nature of the bone allows it to be molded into the extraction socket. The tuberosity should be evaluated with a CBCT survey to determine the maxillary sinus location and the amount of host bone present.
Three-Walled Socket Bone Grafting Technique (Figs. 8a–8d)
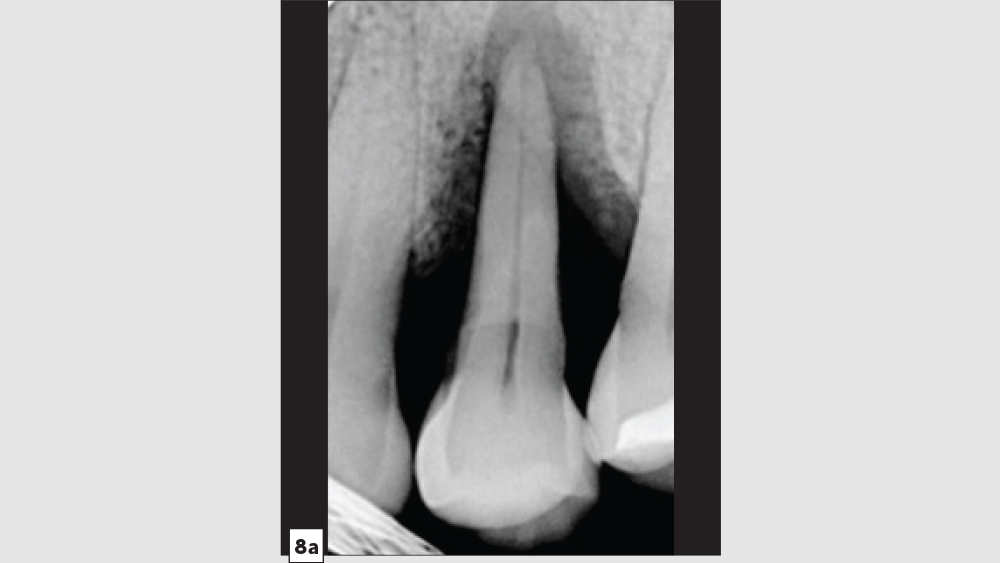
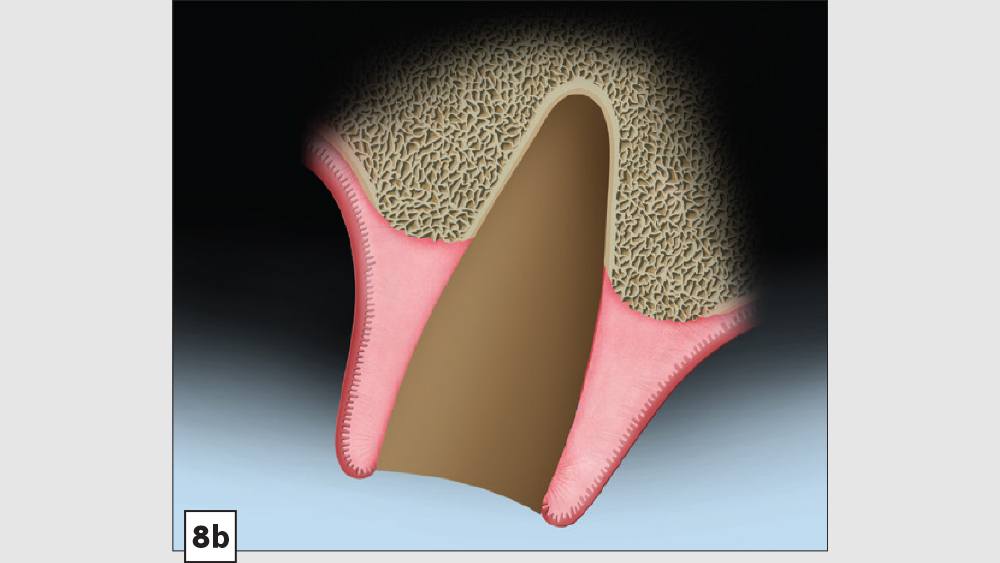
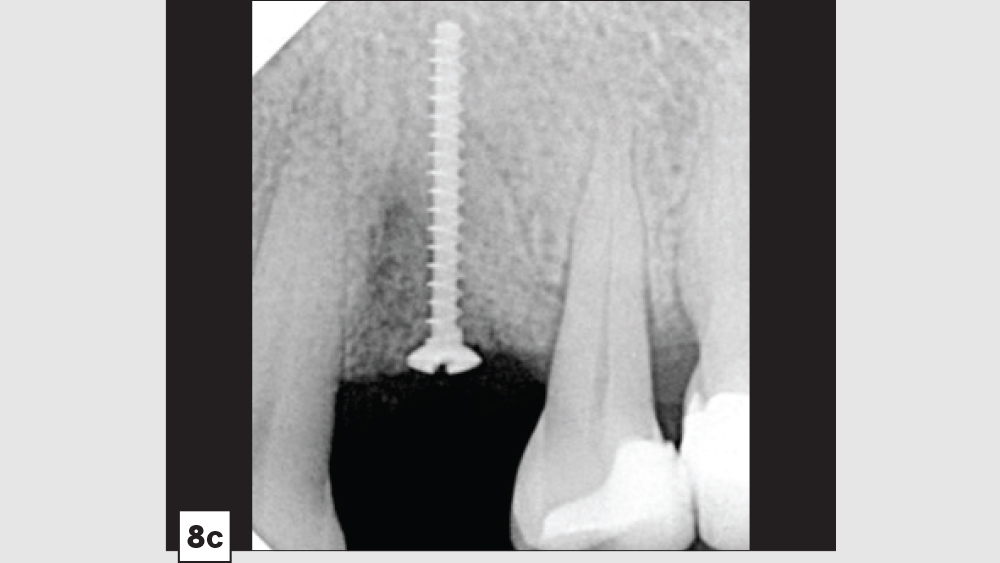
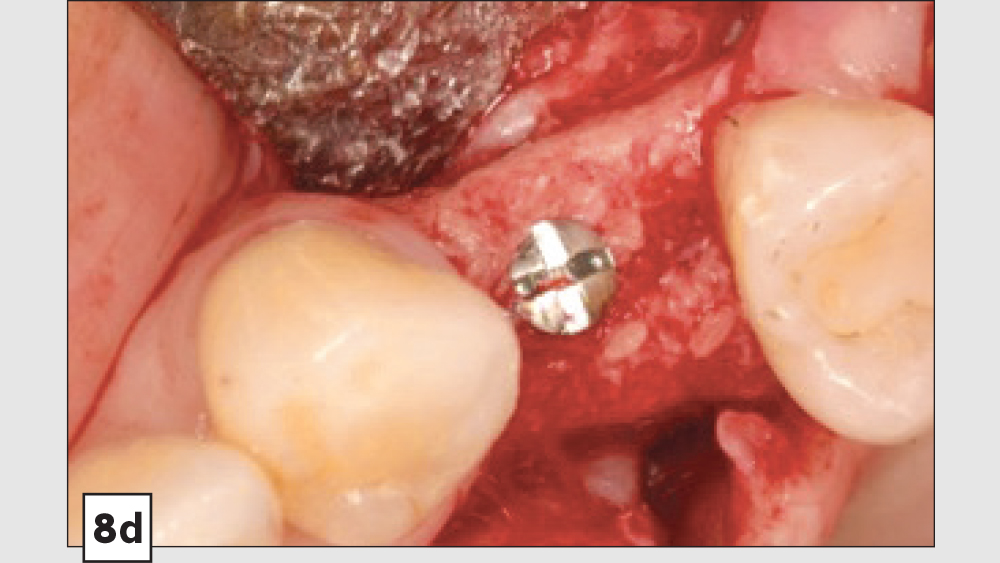
Figures 8a–8d: Three-walled socket: failing tooth with bone loss (8a), example of three-walled socket (buccal and lingual walls missing) (8b), graft with tent screw (8c), post-op healing (8d).
- Reflection: In most cases, these types of defects will require a full-thickness mucoperiosteal flap. Because of the extent of the bony defect, a reflected flap will be beneficial for access, tissue advancement and final closure.
- Preparation: After soft-tissue removal, it is imperative to decorticate the remaining walls of bone. The induced trauma from decortication will initiate the regional acceleratory phenomenon (RAP), which will facilitate early vascularization and initiate the healing process. In most three-walled sockets, tent screws are used to maintain space and increase predictability in the larger defect sites.
- Grafting Material: The autogenous harvested bone should be placed on the host bone defect as the first layer. Particulate allograft is ideally used to fill in any voids and to maintain space. Because autogenous bone is fast-resorbing, the combination of autogenous bone and allograft is beneficial to maintain space and allow sufficient time for bone growth.
- Membrane: In most three-walled sockets, a long-acting membrane (e.g., a collagen cross-linked membrane [Newport Biologics Resorbable Collagen Membrane 4-6] or a PTFE [CytoSurg Non-Resorbable PTFE Membrane]) is recommended. The barrier membrane is modified to encompass the missing socket walls. With these large defects, membrane tacks may be used to prevent any micromovement of the membrane.
- Closure: Closure should be completed with a 3-0 or 4-0 high-tensile suture material (e.g., PGA or PTFE). Care should be exercised to avoid suturing through the membrane.
SOCKET GRAFT HEALING
The grafted socket and soft-tissue healing time is based on many factors, including the following: 1) the number of remaining walls, 2) size of the socket (e.g., molar vs. incisor), 3) type of bone and barrier membrane material, 4) amount of blood supply, and 5) patient-specific factors (e.g., age, systemic diseases, smoking). Usually, soft-tissue healing (epithelization) will occur in approximately three weeks and hard-tissue healing (bone) will take approximately four months. Larger site materials with slower bone turnover may take five to six months. Radiographically, the absence of a cribriform plate may be used as an indicator for adequate healing.
POSTOPERATIVE INSTRUCTIONS
To minimize the possibility of complications and increase the predictability of the socket graft procedures, the following postoperative instructions should be explained to the patient:
- Chlorhexidine (0.12%) may be prescribed for a non-vigorous rinse twice a day.
- Patients should be instructed to avoid contact with the extraction site, and special care should be emphasized not to pull on the lip to evaluate the surgery site. This may lead to incision line opening and delayed healing.
- The interim prosthesis should be evaluated and modified to ensure no contact onto the grafted site.
- Suture removal is approximately 14 days for PGA or PTFE.
Note: In the literature, there is no general consensus on how long d-PTFE membranes are to be left in place. Numerous studies have shown that if the membrane is in place for at least four weeks, dense osteoid tissue will develop, which will prevent the penetration of epithelium and connective tissue.7
AVOIDING SOCKET GRAFTING COMPLICATIONS
The most common complication from socket grafting techniques is inadequate bone graft fill (Figs. 9a, 9b). Care should be exercised to add bone in small increments, especially to ensure that bone is placed in the apical one-third of the socket. A bone packer should be used with good condensation to avoid air spaces. Additional complications include overfilling of the graft material (Fig. 10). Bone graft material should be placed at the level of the bony crest to allow space for the membrane. If excess bone graft material is condensed above the bone crest, delayed healing will occur.
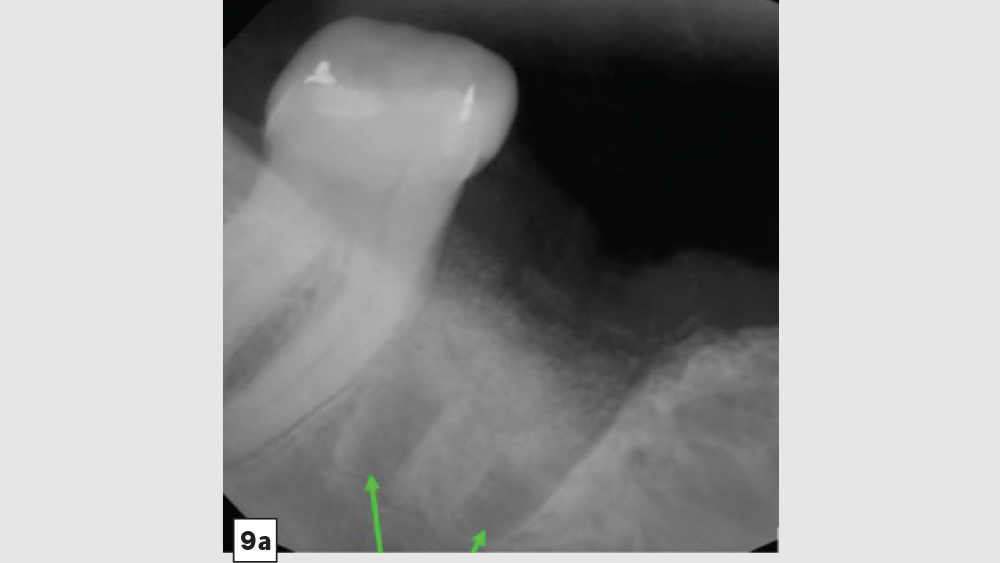
Figures 9a, 9b: Inadequate socket grafting with large voids in apical one-third (9a), inadequate bone fill with resultant air space (9b).
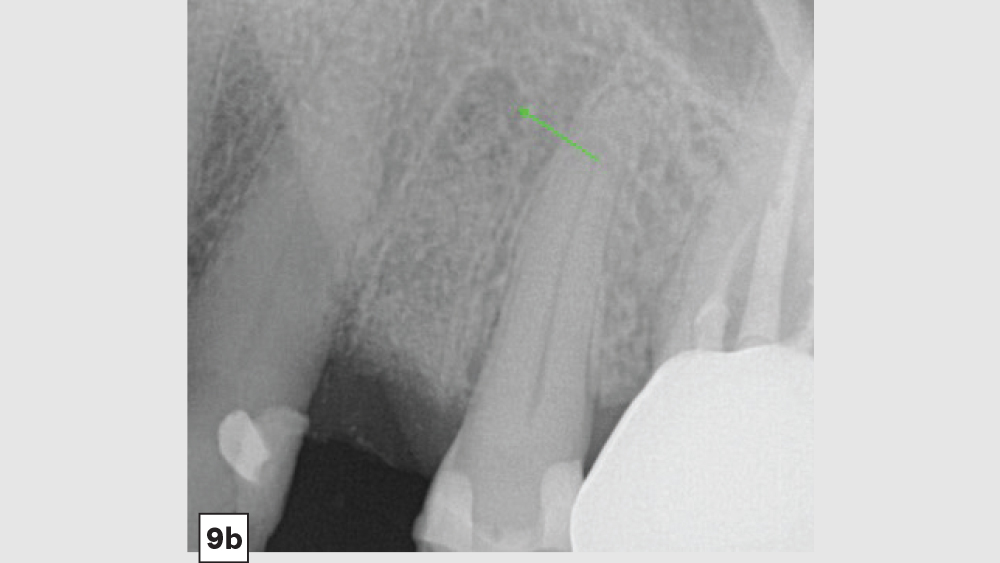
Figures 9a, 9b: Inadequate socket grafting with large voids in apical one-third (9a), inadequate bone fill with resultant air space (9b).
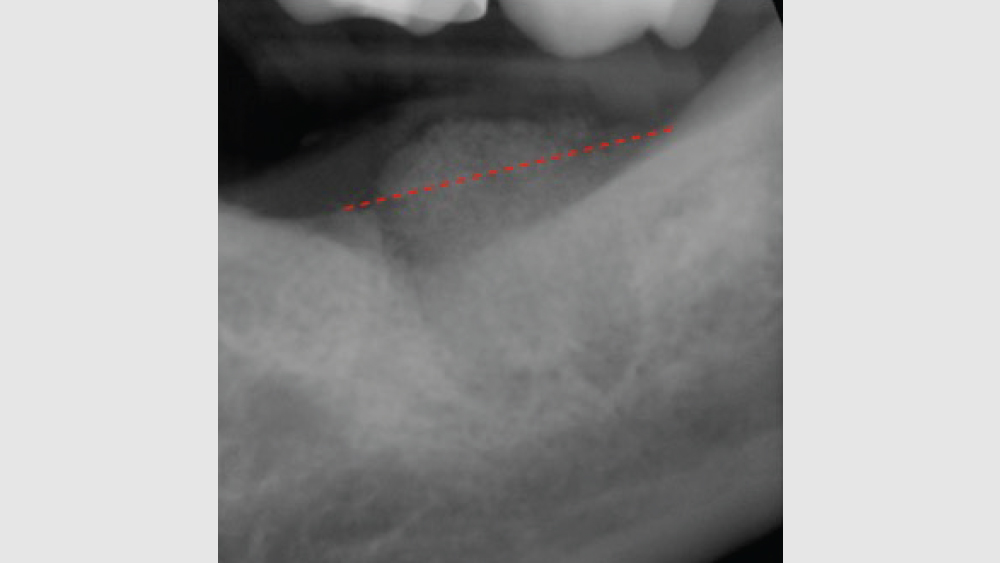
Figure 10: Excess bone graft above the level of the bony crest.
To decrease postoperative infection, especially in situations where postoperative infection is high (i.e., immunocompromised patients, presence of infectious bacteria, smokers, etc.), the addition of a local antibiotic to the bone graft material is recommended. Although many antibiotics are used in dentistry, the author recommends the use of cefazolin powder, such as ANCEF® (GlaxoSmithKline; Research Triangle Park, N.C.), to prevent postoperative infectious episodes. Cefazolin has numerous advantages when used as a local antibiotic, as the literature has shown it has no adverse effects on osteoblasts or bone growth,8 and leads to an increase in alkaline phosphatase activity,9 as well as increased prevention of implant-related infections.10 Cefazolin (1 g) should be reconstituted with 2.0 cc of sterile saline (500 mg/mL). Usually, 1.0 cc is added to the bone of the surgical site.
CONCLUSION
Treating all extraction sockets with the same surgical protocol will result in increased morbidity and a decreased amount of predictable bone growth. Therefore, the clinician must first identify the type of extraction socket and then treat with the correct surgical protocol as discussed in this article. When the proper protocol is utilized, socket grafting is a beneficial and predictable procedure for the clinician and patient.
Available CE Course
References
- ^ Becker W, Becker BE, Caffesse R: A comparison of demineralized freeze-dried bone and autologous bone to induce bone formation in human extraction sockets. J Periodontol. 1994 Dec;65(12):1128-33.
- ^ Piattelli, A., Scarano A, Corigliano M, Piattelli M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: a histological and histochemical study in man. Biomaterials. 1996 Jun;17(11):1127-31.
- ^ Skoglund A, Hising P, Young C. A clinical and histologic examination in humans of the osseous response to implanted natural bone mineral. Int J Oral Maxillofac Implants. 1997;12(2):194-9.
- ^ Araújo M, Linder E, Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res. 2009 Jan;20(1):1-6.
- ^ Bartee BK. Extraction site reconstruction for alveolar ridge preservation. Part 2: membrane-assisted surgical technique. J Oral Implantol. 2001;27(4):194-7.
- ^ Elian N, Cho SC, Froum S, Smith RB, Tarnow DP. A simplified socket classification and repair technique. Pract Proced Aesthet Dent. 2007 Mar;19(2):99.
- ^ Greenstein G, Carpentieri JR. Utilization of d-PTFE barriers for post extraction bone regeneration in preparation for dental implants. Compend Contin Educ Dent. 2015 Jul-Aug;36(7):465-73.
- ^ Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effects of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996 Dec;(333):245-51.
- ^ Li H, Ogle H, Jiang B, Hagar M, Li B. Cefazolin embedded biodegradable polypeptide nanofilms promising for infection prevention: a preliminary study on cell responses. J Orthop Res. 2010 Aug;28(8):992-9.
- ^ Park ES, Maniar M, Shah JC. Biodegradable polyanhydride devices of cefazolin sodium, bupivacaine, and taxol for local drug delivery: preparation, and kinetics and mechanism of in vitro release. J of Controll Release. 1998 Mar 2;52(1-2):179-89.



